Inclusion Body Myositis (IBM)
A.) IBM Overview
Sporadic inclusion body myositis (IBM) is the most common acquired muscle disease in adults over the age of 50, yet the underlying cause of the disease is unknown, and there are no disease-modifying therapies. The robust endomysial inflammation with autoreactive CD8+ T-cells invading healthy-appearing myofibers in addition to an increased association of IBM with specific HLA haplotypes and other autoimmune diseases suggests that IBM is primarily an autoimmune disease. However, an association with aging, lack of response to immunotherapy, and presence of pathological features such as TDP-43 cytoplasmic aggregation seen in neurodegenerative diseases suggest the immune response may be secondary to myodegeneration. Thus, the relationship between inflammation, inclusions, TDP-43 pathology, and degeneration in IBM is poorly understood.
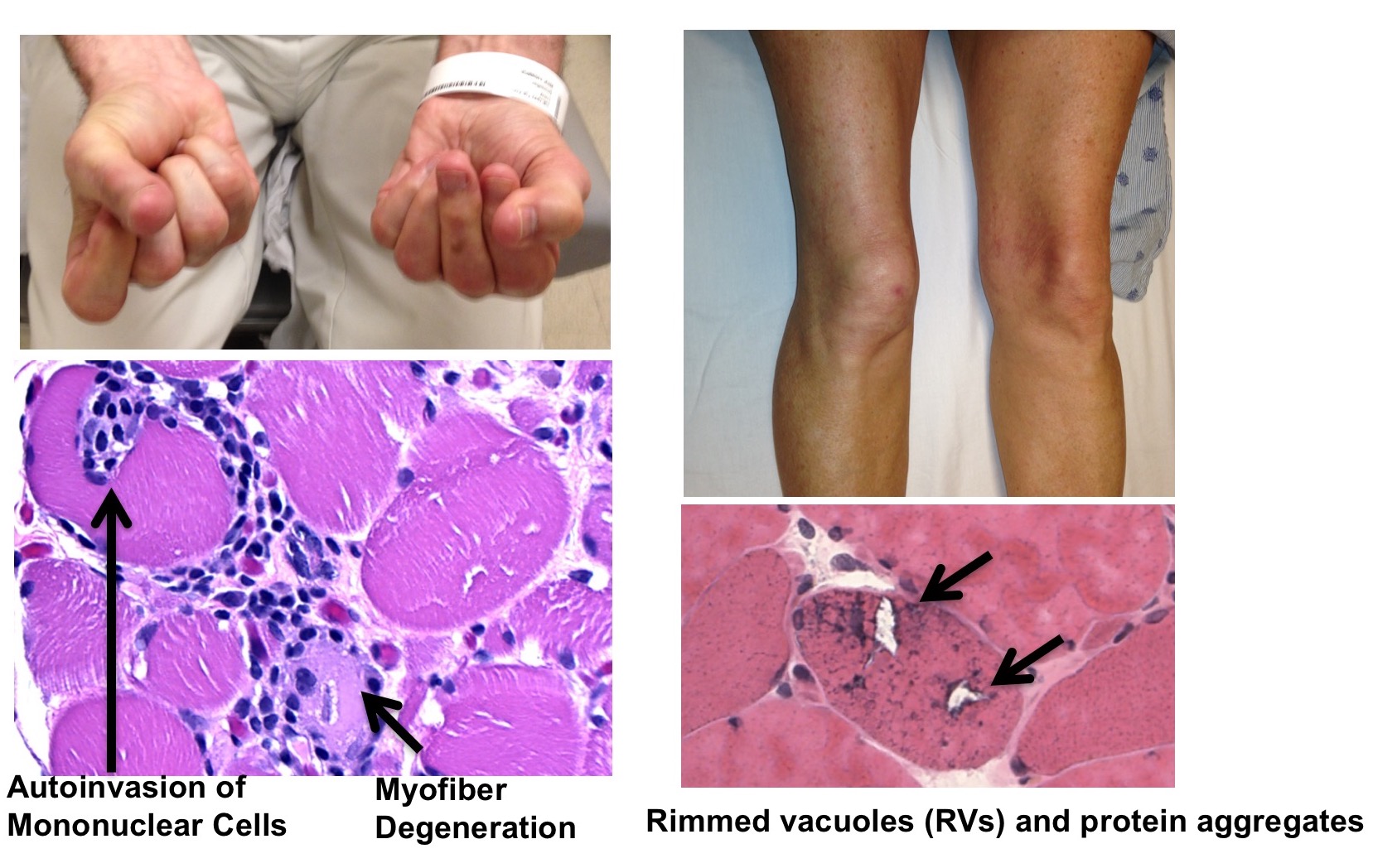
B.) Using a Xenograft model to study the pathogenesis of Inclusion Body Myositis

The mechanisms underlying the pathogenesis of Inclusion Body Myositis (IBM) have long eluded researchers. Part of this difficultly arises from the unique combination of both inflammatory and degenerative features observed within the muscle of patients. To better understand the roles these features play in the development of IBM, our lab has developed a xenograft model of IBM. The aims of this project are:
- To characterize a novel mouse xenograft model of sporadic IBM.
- To determine whether splicing repression is a major role of TDP-43 in skeletal muscle.
- To test novel therapeutic targets in a xenograft model of IBM.
We’ve found that IBM muscle regenerates robustly in immunocompromised host mice and recapitulates several features of the human pathology.
Like many neurodegenerative diseases, IBM has been classified as a TDP-43 proteinopathy characterized by nuclear clearing and cytoplasmic aggregation of the RNA-binding protein TDP-43. We aim to test whether this mislocalization of TDP-43 underlies muscle degeneration in IBM.
There are no clinically effective treatments for patients diagnosed with IBM, and the lack of animal models for this disease has been a fundamental obstacle for the development of therapies. We hypothesize that this xenograft model will allow us to carry out preclinical testing of potential therapeutics.
Publications
- Britson KA, Black AD, Wagner KR, Lloyd TE. Performing Human Skeletal Muscle Xenografts in Immunodeficient Mice. J Vis Exp. 2019 Sep 16;(151). doi: 10.3791/59966.
- Hanna, MG, Badrising UA, Benveniste O, Lloyd TE, Needham M, Chinoy H, Aoki M, Machado PM, Liang C, Reardon KA, et al, and the RESILIENT Study Group. Safety and efficacy of intravenous bimagrumab in inclusion body myositis: a phase 2b, randomised, double-blind, placebo-controlled study (RESILIENT). Lancet Neurology. 2019. 8(9):834-844. PMID 31397289.
- Vogler TO*, Wheeler JR*, Nguyen ED, Hughes MP, Britson KA, Lester E, Rao B, Betta ND, Whitney ON, Ewachiw TE, Gomes E, Shorter J , Lloyd TE, Eisenberg DS, Taylor JP, Johnson AM , Olwin BB*, Parker R*. Amyloid-like TDP-43 myo-granules associate with 1 sarcomeric RNAs during skeletal muscle formation. Nature Article Nature. 2018 Nov;563(7732):508-513. PMID: 30464263
- Britson KA, Yang SY, Lloyd TE. New Developments in the Genetics of Inclusion Body Myositis. Curr Rheumatol Rep. 2018 Apr 2;20(5):26. PMID:29611059
- Lloyd TE, Fernandez IP, Michelle EH, Christopher-Stine L, Pak K, Sacktor N, and Mammen AL. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology. 2017; 88(15):1454-1460.
- Amici DR, Pinal-Fernandez I, Mázala DA, Lloyd TE, Corse AM, Christopher-Stine L, Mammen AL, Chin ER. Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis. Acta Neuropathol Commun. 2017; 5(1):24.
- Güttsches AK, Brady S, Krause K, Maerkens A, Uszkoreit J, Eisenacher M, Schreiner A, Galozzi S, Mertens-Rill J, Tegenthoff M, Holton JL, Harms MB, Lloyd TE, Vorgerd M, Weihl CC, Marcus K, Kley RA. Proteomics of rimmed vacuoles define new risk allele in inclusion body myositis. Ann Neurol. 2017 Feb;81(2):227-239.
- Lloyd TE, Christopher-Stine L, Pinal-Fernandez I , Tiniakou E, Petri M, Baer A, Danoff S, Pak K, Casciola-Rosen L, Mammen AL. Cytosolic 5’-nucleotidase 1A is a common target of circulating autoantibodies in several autoimmune diseases. Arthritis Care Research 2016; 68:66-71.
- Lloyd TE*, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA.* Evaluation and Construction of Diagnostic Criteria for Inclusion Body Myositis. Neurology. 2014; 83:426-33.
- Roda RR, Schindler A, Blackstone C, Mammen AL, Corse AM, Lloyd TE. Laing Distal Myopathy Pathologically Resembling Inclusion Body Myositis. Annals of Clinical and Translational Neurology 2014:1053-8.
- Lloyd TE. Novel therapeutic approaches for inclusion body myositis. Current Opinion in Rheumatology. 2010; 22(6): 658-64.